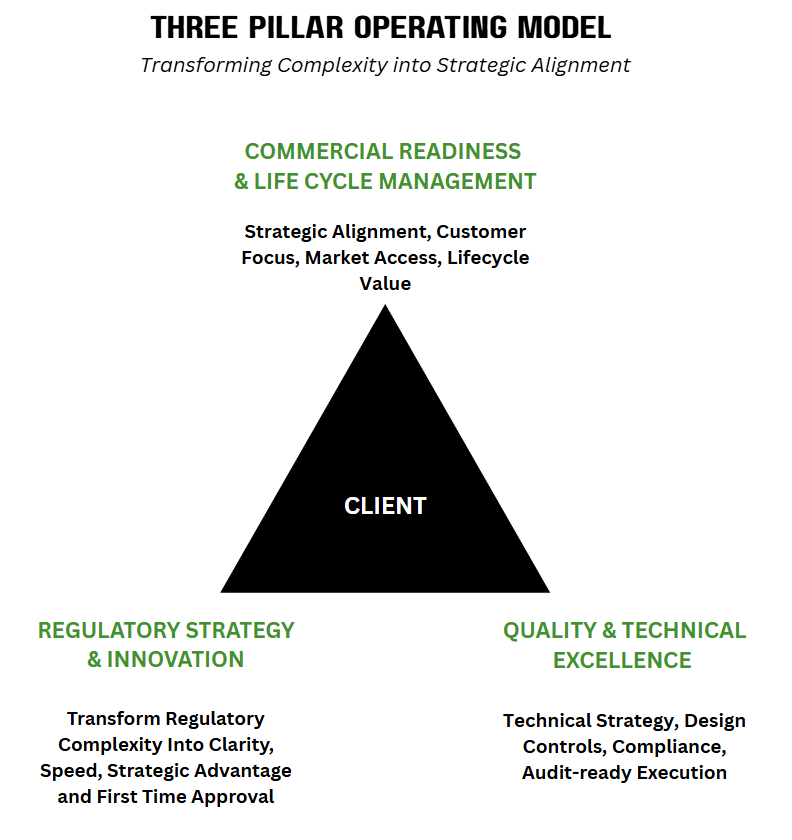
We believe true market success comes when regulatory, technical, and commercial strategies work in harmony.
Our Three‑Pillar Operating Model ensures this alignment:
Regulatory Strategy & Innovation – Simplifying regulatory complexity into clarity, speed, and first-time approvals, creating strategic advantage and confidence.
Quality & Technical Excellence – Delivering robust technical strategy, design controls, compliance, and audit-ready execution to ensure safe and reliable outcomes.
Commercial Readiness & Lifecycle Management – Driving strategic alignment, customer focus, market access, and lifecycle value to maximize impact and long-term success.
At the centre of everything is the client, with our model enabling faster decisions, protected value, and lifecycle readiness — supporting the safe, timely delivery of products to patients across the US, EU and Global markets.
How We Work
Diagnose
Identify strategic challenges through focused workshops and stakeholder interviews. Uncover cross-functional risks, lifecycle bottlenecks, and alignment gaps across commercial, regulatory, and technical domains—laying the foundation for enterprise-level transformation.
Design
Craft tailored solutions in partnership with your teams, aligned with your organisation’s context, culture, and ambitions. Guided by CPMD’s Three-Pillar Operating Model, create coherence across commercial readiness, regulatory strategy, and quality/technical excellence—practical, actionable, and strategically robust.
Deliver
Embed and scale solutions with disciplined implementation support and targeted capability building. Ensure new ways of working deliver portfolio-wide impact, sustained adoption, and full ownership by your teams for lasting results.